
“My laboratory has recently developed new technologies enabling us to study the human retina, to understand its functional architecture and disease mechanism in its various cell types, and so to develop therapies”, Botond Roska reports. Spe-cific understanding of both healthy and disease-affected human retinas is therefore essential. Only a few animal models of visual diseases reproduce the pathology found in humans. Despite the large number of cortical neurons involved in vision, most blinding diseases originate in the retina and are cell-type specific. The information then flows from the retina to the brain – via the thalamus to a number of cortical areas. The photoreceptors process images via retinal circuits built from more than a hundred different cell types. Photoreceptor cells in the retina capture the light that falls on the eye and transduce it into neuronal activity. Understanding how the retina transforms images from the outside world into signals that the brain can interpret is crucial for developing therapies for blinding retinal diseases. Vision starts in the retina, a powerful biological computer in our eyes.

“The grant also allows us further to grow our outstanding team of molecular researchers and clinicians, combining their strengths by working hand in hand daily.” About the HURET project
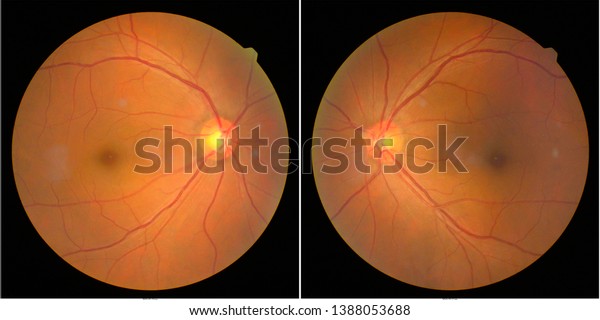
This funding boosts our studies of disease mechanisms and contributes to developing therapies for restoring vision faster”, says Botond Roska. It will enable our IOB teams to take major steps to accelerate development of novel therapies for currently untreatable ophthalmic diseases. “I am very happy to be awarded this significant funding. The grant call was highly competitive: the ERC received more than 1880 submissions. The award is for a project called HURET: The human retina at single cell resolution: functional architecture, disease mechanism and therapy development. Vision ACTion and its partners are developing diagnostics and therapeutics for both nAMD and GA, and we are exploring the factors that determine progression from early to late-stage disease.Professor Botond Roska, Co-Director of the Institute of Molecular and Clinical Ophthalmology Basel (IOB) and Professor at the University of Basel, Faculty of Medicine, has won a € 2.5 million Advanced Grant from the European Research Council (ERC). By 2030 there are expected to be about 1.44 million Australians with early AMD, and 215,000 who are visually impaired in both eyes by later-stage AMD. As the name implies the risk of AMD rises with age, especially over age 65. The ratio of nAMD to GA patients is about 2:1. There are treatments for nAMD but little for GA. These are sometimes called Dry and Wet AMD, although older nomenclature also refers to the earlier drusen-only phase as Dry AMD. At certain point patches of retina go into one of two states: death of the retinal layers called geographic atrophy (GA), or growth of malformed and destructive leaky blood vessels called neovascular AMD (nAMD).

In early AMD this garbage begins to accumulate, forming deposits called drusen that sit within the retinal layers.Īs AMD progresses these drusen become larger and more numerous. This causes a potential garbage recycling problem. To keep in top shape a substantial proportion of older layers get discarded and replaced every day. Inside each of the 200 million rod and cone cells are stacks of hundreds of light-absorbing layers. The remaining retinal layers and parts of the brain work-up those signals to create our visual perception. Photoreceptors reside in the deepest layer of the retina, and include the cone cells (which give us colour vision) and the rods (which give us our night vision). The photoreceptor cells of the retina capture light and transform it into a signal which can be interpreted by neurons in the brain.


 0 kommentar(er)
0 kommentar(er)
